NK cell therapy
NK and cells
NK (natural killer) cells are a type of lymphocyte that play a central role in the innate immunity (defense mechanism) that we are born with. NK cells patrol the body and have the special ability to instantly recognize abnormal cells such as "virus-infected cells" and "cancer cells" and attack them without hesitation (autonomously).
It is said that 5,000 cancer cells are born in our bodies every day, but NK cells suppress the growth and onset of cancer by destroying individual cancer cells.
However, as the number and function of NK cells decline with age (NK activity declines), the ability to suppress cancer cells weakens, making the body more susceptible to developing cancer.
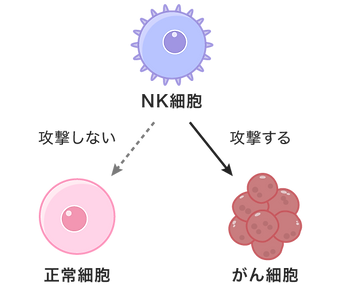
NK cells do not receive instructions from anywhere.
Attacking cancer cells
What is NK cell therapy?
NK cell therapy is a treatment in which NK cells (natural killer cells) are isolated from the patient's own blood, cultured to grow, and then returned to the body via intravenous infusion.
This will result in more activated NK cells circulating around the body than usual, which is expected to result in a significant improvement in immunity. Boosting immunity will strengthen the body's defenses against cancer cells, viruses, and other such diseases.

Blood collection

Centrifugation




Start of culture
Expanding NK cells in culture
Administered intravenously to patients
Symptoms and concerns that can be expected to improve with NK cell therapy
*The therapeutic effects of regenerative medicine vary from person to person.
Cancer prevention and recurrence prevention
Cancer prevention
Preventing cancer recurrence
Preventing cancer metastasis
*Can be used in combination with other cancer treatments (surgery, anticancer drugs, radiation therapy, etc.)
Diseases associated with weakened immune system
Chronic Fatigue Syndrome (CFS)
Long-lasting colds and flu
Infections due to chronic immune deficiency
Improvement of allergic diseases
Atopic dermatitis
Hay Fever
Chronic urticaria, etc.
Suppression of viral infections
Herpes virus infections
Virus-related diseases
Chronic Hepatitis B and C, etc.
Preventive medicine and recovery promotion
Maintenance of physical fitness and preventive treatment for chronic diseases
Accelerating recovery after surgery
Improves age-related immune decline
Age-related decline in immunity
Age-related diseases, etc.
At our hospital, we can treat the following conditions using adipose tissue-derived mesenchymal stem cells: endometrium, wrinkles and age spots (age-related changes in the skin), osteoarthritis, and pain (chronic pain).
Project number: "Treatment for endometrial regeneration failure using autologous adipose tissue-derived mesenchymal stem cells"
Project number: "Treatment using autologous adipose-derived stem cells for age-related changes in the skin, such as wrinkles and sagging skin"
Project number: "Treatment for osteoarthritis using autologous adipose-derived stem cells"
Project number: "Treatment for chronic pain using autologous adipose-derived stem cells"

NK cell therapy process
1. Doctor's examination and counseling
2. Blood Tests
When you start treatment, a blood test will be done.
Blood samples can be taken on the day of your appointment.
*Please let us know if you would like to attend on a different day.
3. Peripheral blood collection *One week after blood test
We will collect blood to culture NK cells.
4. NK cell culture *This takes about 2 months
5. Administration of NK Cells
NK cells are administered intravenously through a peripheral vein over a period of approximately 30 minutes.
6. Follow-up
We will conduct examinations 1, 3, 6, and 12 months after administration of NK cells to check whether the therapeutic effect is being obtained safely and effectively. We will also conduct tests using various testing equipment as necessary.
"Type 2 Regenerative Medicine Provision Plan Number"
The suitability of NK cell therapy is strictly examined by the Specific Certified Regenerative Medicine Committee, which is certified by the Ministry of Health, Labor and Welfare. Treatment is only possible if a treatment plan is submitted to the Ministry of Health, Labor and Welfare and a plan number is obtained after the therapy is deemed appropriate.
GINZA NOA CLINIC has gone through the proper process to report to the Ministry of Health, Labor and Welfare:
We have submitted a "Type 2 Regenerative Medicine Provision Plan" and obtained a plan number.

Cell Processing Center (CPC) within the hospital
Our hospital is equipped with a cell culture processing room (CPC). Cell culture and PRP production are possible in our in-house CPC, which has been approved by the Ministry of Health, Labor and Welfare. In addition, our experienced cultivators are equipped with the latest equipment, including PCR machines and measuring instruments, to provide patients with stable, high-quality cells. Inside the CPC, we have established a hygiene management system that complies with the P2 level set by the government, including thorough sterilization with germicidal lamps and prevention of backflow of air inside and outside with HEPA filters.
Our CPC has been assigned a facility number (FC3250034) by the Ministry of Health, Labour and Welfare in accordance with the Act on Ensuring the Safety of Regenerative Medicine, etc.

Treatment in a private room
Treatment is carried out in a private room to ensure patient privacy.



Risks and Side Effects
side effects
Since NK cell therapy uses the patient's own cells, the risk of rejection or serious side effects is considered to be extremely low.
Possible side effects that have been reported include the following:
Fever
Fatigue
If you have any concerns or changes in your physical condition, please consult your doctor immediately. Our clinic provides safe and secure regenerative medicine through pre-treatment counseling and follow-up after treatment.
About this treatment
Unapproved drugs, etc.: The drugs used in this treatment are unapproved drugs that have not been approved under the Pharmaceuticals and Medical Devices Act.
Acquisition route, etc.: This drug is dispensed legally in-house (with some outsourcing).
Availability of other drugs approved in Japan: There are no other drugs approved in Japan with the same ingredients or properties.
Information on safety in other countries: No significant safety issues have been reported in other countries.
These products are not eligible for relief under the Pharmaceutical Adverse Reaction Relief System or the Biological Product Infection Relief System.
Pricing & Plans
This treatment is an elective medical treatment. Since elective medical treatment is not covered by the public insurance system, you will be responsible for all medical expenses.
For patients overseas
If patients residing overseas wish to visit our hospital for medical examination or consultation, in order to ensure smooth and safe medical treatment, as a general rule, they will be asked to complete the procedures through a medical coordination company.
There are separate fees for those coming to Japan from overseas for treatment.
For more information, please contact our International Department.